Abstract
BACKGROUND :
Spur cell hemolytic anemia (SCHA) is a rare, acquired, non-immune hemolytic anemia of decompensated cirrhosis resulting from abnormal lipid composition of the red cell membrane. Treatment is limited to red cell transfusion. Data describing prognostic impact, outcomes of liver transplant, and clinical hematologic characteristics of SCHA are absent or limited; published data on SCHA are limited to single-patient case reports and small single-center case series. The independent prognostic impact of SCHA on patients with cirrhosis remains unclear, and it is not known if hemolytic anemia severity has a significant impact on survival of these patients. As a result, SCHA is not formally considered during liver transplant evaluation, and patients do not receive MELD exception points for this diagnosis.
METHODS :
We performed a multicenter, 24-year observational cohort study of patients with SCHA, retrospectively analyzing hepatic and hematologic parameters, independent predictors of mortality, and long-term outcomes of liver transplant. Strict diagnostic criteria for SCHA were applied, requiring all of the following: (1) decompensated cirrhosis; (2) anemia; (3) objective laboratory evidence of hemolysis; (4) no alternate contributing cause of hemolysis or acanthocytosis; and (5) chart documentation of a confirmed diagnosis of SCHA incorporating peripheral blood film examination. The primary outcome was mortality at 3 months after date of SCHA diagnosis. The impact of hemolytic parameters on 3-month mortality was evaluated utilizing multivariable logistic models. Observed mortality vs. expected mortality (per MELD-Na score and Child-Turcotte-Pugh class) was compared using standardized mortality ratios. Given the limited survival of this population, red cell transfusion dependence was defined as 4 or more units of red cells transfused during the 60-day peri-diagnostic period.
RESULTS :
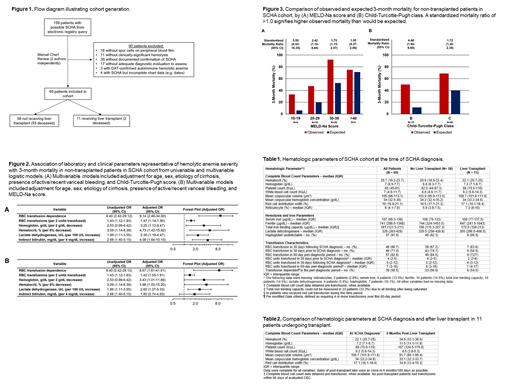
Patients: 69 patients with SCHA were included (FIGURE 1). The median (interquartile range) age was 53 (42-59) years; 46.4% were female, and 11 (15.9%) received liver transplant. Alcohol contributed to the etiology of cirrhosis in 53 patients (76.8%). The median (IQR) survival from SCHA diagnosis of patients not receiving liver transplant was 58 (23-113) days. 39 patients (56.5%) were red cell transfusion-dependent. Hematologic parameters are described in TABLE 1.
Outcomes of Liver Transplant: All 11 patients undergoing transplant had rapid and complete resolution of SCHA, with an improvement in median hematocrit from 22.1% to 34.6% post-transplant (P=0.001) (TABLE 2) and excellent post-transplant outcomes, with 9 patients still alive after 6.1 years median follow-up.
Independent Predictors of Mortality in SCHA: In multivariable logistic models adjusting for age, sex, etiology of cirrhosis, active/recent variceal bleeding, and Child-Turcotte-Pugh score, transfusion dependence had an OR for 90-day mortality of 9.14 (95% CI, 2.46-34.00) and reduced pre-transfusion hematocrit had an OR of 4.73 (95% CI, 1.42-15.82) per 6% decrease; increased red cell transfusion requirement, reduced hemoglobin, increased lactate dehydrogenase, and increased indirect bilirubin were also independently predictive of higher 90-day mortality (FIGURE 2).
Performance of MELD-Na and Child-Turcotte-Pugh Scores in Estimating 90-Day Mortality: MELD-Na and Child-Turcotte-Pugh scores consistently significantly underestimated 90-day mortality, with standardized mortality ratios (SMRs) >1 across all scores/classes [MELD-Na 20-29, SMR 2.42 (1.18-4.44); Child-Turcotte-Pugh class B, SMR 4.46 (1.64-9.90)], FIGURE 3.
CONCLUSIONS :
In this largest study of SCHA to date, SCHA was associated with substantial excess mortality than was predicted by MELD-Na or Child-Turcotte-Pugh scores. Several clinical and laboratory parameters of hemolytic anemia severity, including transfusion burden, hemoglobin, and markers of hemolysis were each independent predictors of 90-day mortality in SCHA. Despite its unique morbidity as a complication of decompensated cirrhosis, outcomes of liver transplant were excellent in all 11 patients undergoing this intervention, with total resolution of SCHA and no evidence of recurrence. These findings should promote greater awareness of SCHA as a clinical entity and broader consideration of MELD exception points for afflicted patients when transplant-eligible.
Al-Samkari: Novartis: Consultancy; Amgen: Research Funding; Argenx: Consultancy; Rigel: Consultancy; Dova/Sobi: Consultancy, Research Funding; Agios: Consultancy, Research Funding; Moderna: Consultancy.